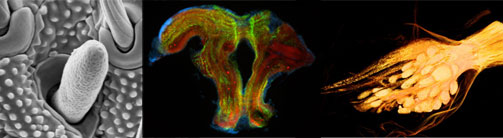
Research
Emerging research topics
- Understanding the combined effects of fluctuations of multiple environmental drivers on developmental performance of and organogenesis in marine invertebrate larvae
- Understanding environmentally induced and stochastic developmental components of phenotypic variation in arthropod nervous systems
- Crustacean metamorphosis: development of the sensory, central nervous, and neuromuscular system in relation to behavioural changes during metamorphic larval development.
Current research topics
- Functional morphology, development and evolution of crustacean olfactory systems.
- Transition from sea to land: adaptations of the central olfactory pathway in land hermit crabs
- “Neurophylogeny”: exploring structure, development and evolution of the central, peripheral and neuromuscular nervous system of Arthropoda for understanding the evolution of nervous systems in general.
- Persistent neurogenesis in the brain of ADULT crustaceans
Past research projects
- Spatial and temporal patterns of proliferation of neuronal stem cells in the crustacean ventral nerve cord
- Development of the crustacean visual system and insights into arthropod phylogeny
- Individually identified neurons and insights into arthropod phylogeny
- Comparative anatomy of the neuromuscular system of arthropod limbs
- Structure and development of the nervous system in arrow worms
A major part of our research seeks to achieve new insights into the structure and evolution of the nervous system in the Arthropoda. To reconstruct structural transformations and shed light on the evolution of the nervous system across the extremely diverse and disparate arthropod taxa, it is indispensable to use a comparative approach. In our group, a strong focus is placed on representatives of the Crustacea and some Chelicerata. Specifically, we are interested in the architecture of the brain, in particular in the neural circuitry underlying the chemosensory pathway.
Further, we investigate the embryonic and larval development of the crustacean and chelicerate nervous systems. Some of our ontogenetic studies extend all the way into the adult, deciphering spatial and temporal cell division patterns related to persistent adult neurogenesis in specific areas of brain and ventral nerve cord, such as, for instance, the central olfactory pathway.
Moreover, we are also interested in the neuroethology of arthropods and study selected behaviors, such as odor tracking or shell-choice behavior, as well as learning in hermit crabs. One intriguing question relates to the neural substrate for the animals’ behavioral repertoire and whether persistent neurogenesis and integration of new neurons into association centers of the adult brain are involved in learning processes.
Another focus lies on the morphology of fossil arthropods – including insects in amber – to find evidence for behavioral aspects.
To visualize and document the various aspects of adult morphology, neuroanatomy and neurodevelopment, we employ a range of state-of-the-art techniques, including immunohistochemistry, in-situ hybridization, proliferation marker assays, confocal laser-scanning microscopy, X-ray microscopy, and computer-aided 3D reconstruction.
Our research is supported by several funds from the Deutsche Forschungsgemeinschaft(DFG).
In the framework of the DFG funded Research training Group 2010 RESPONSE, we analyze the effect of abiotic stressors (temperature and salinity) on larval development of crustaceans in a cooperation with the Alfred-Wegener-Institute Helmholtz-Centre for Polar- and Marine Research.
Ecomorphology and biomechanics of raptorial forelegs in praying mantises (Insecta: Mantodea) - (DFG: BU3169/3-1)
by Dr. Sebastian Büsse
How did praying mantises (Mantodea) get so successful as ambush predators in various habitats by using prehensile raptorial forelegs and what can we learn from it?
This main question will be investigated by looking at three sub-questions:
How did Mantodea adapt to different food sources and ecological niches–morphologically and behaviourally?
Mantodea live in various habitats; from the canopy of a tree over its bark towards the forest floor–from wet rainforests up to dry deserts. This comprises very different environmental constraints, very different compositions of the respective biocoenosis and thus a very different food and predator spectrum (and so on, and so on). However, these very different mantis habitats have one thing in common–Mantodea as arthropod key predators. In this project, I want to highlight the adaptations of the prey-capturing apparatus and the predatory strike of praying mantises that made them so successful. This will highlight the adaptations, like the set-up of spines and setae on the raptorial forelegs, changes in ex- and intrinsic musculature or the predatory strike itself, that help the different ecotypes to proceed in their respective habitats.
Are there biomechanical principles of terrestrial fast-motion prey grasping in general and for different types of prey in particular?
A problem can often be solved in different ways. But is there an underlying principle or is one solution better than the other for a specific problem? Here I want to foster our understanding of how the interplay of structure and behaviour allows mantises to capture very different prey items, and what mantises that specialise in a particular type of prey (e.g. flying or ground-dwelling prey), do differently than generalists. This will help to identify the key characters that are needed to catch a certain type of prey more successfully.
Can some of these principles be used for biomimetics?
These extracted principles, together with detailed information on the structure and biomechanics of the raptorial forelegs in Mantodea, will help to improve or completely create new ideas, for example in robotic grasping devices; like robotic grasping systems employing the mentioned lever and ratchet system. Furthermore, these robotic implications will build an artificial platform for biological experiments to further deepen our knowledge around the biological system(s).
DFG-funded project in PP 2205 „Evolutionary Optimisation of Neuronal Processing“ Theme A: convergent evolution or evolutionary specialisation of shared core circuits
Applicants: Prof. Dr. Steffen Harzsch (University of Greifswald), Dr. Jürgen Rybak (MPI Chemical Ecology, Jena), Prof. Dr. Martin Nawrot (University of Cologne)
According to all recent molecular phylogenies, insects must be regarded as a highly successful terrestrial animal group that originated from marine crustaceans. The main chemosensory organ of both taxa for distance olfaction is the most anterior pair of head appendages, the deutocerebral antennae. Olfactory sensory neurons associated with sensilla on the antennae project their axons into the brain’s primary olfactory centres, the bilaterally paired antennal lobes (insects)/olfactory lobes (crustaceans). There, sensory olfactory afferents synapse with two classes of olfactory interneurons (OSNs), the local olfactory interneurons (LNs) and the olfactory projection neurons (PNs), within specialized neuropil compartments, the olfactory glomeruli. Although the principle wiring pattern within the glomeruli of crustaceans and insects share many similarities, nevertheless there also exist pronounced difference of these olfactory core circuits. Therefore, this system can be seen as an ideal playground for those interested in analysing evolutionary diversification of neuronal core circuits. For example, our results from phase 1 of the PP strongly suggest that what is currently described as „canonical olfactory system organization” e.g. in the benchmark system Drosophila melanogaster likely is a derived character state that evolved from a distributed representation / combinatorial coding, and more and more examples from crustaceans and from within the insects emerge as deviations from this pattern. By comparing insects and crustaceans we can study the end point of past evolutionary optimization processes. This project brings together two experts on the functional and evolutionary morphology of crustacean (HAR) and insect (RYB) olfactory system and a computational neuroscientist with a strong background in arthropod olfactory systems (NAW). In the prolongation proposal, we want to continue our successful collaboration from phase 1 of the PP and gain more detailed insights into the neuroanatomy of the central olfactory pathway in non-model insects and crustaceans beyond the well studied model system D. melanogaster. By quantifying the numbers of the involved neuronal elements (olfactory sensory neurons, local olfactory interneurons, projection neurons, olfactory glomeruli) and by analysing projectomic characteristics of the OSN to PN to output pathway (mushroom bodies) we will gain additional insights into divergence versus convergence and evolutionary specialization of the central olfactory pathways in arthropod lineages. Furthermore, by obtaining ultrastructural data we will continue to understand the evolutionary optimization of the connectivity of OSNs to PNs. Finally, by further evolving a mathematical model of information processing within olfactory glomeruli of different arthropod lineages as developed in phase 1, we want to further understand the existing differences of olfactory coding mechanisms in insects versus crustaceans.
Project B3 - Effects of environmental stress on neurogenesis in crustaceans
DFG-funded project(s) B3 within the research training group (RTG 2010) RESPONSE
Applicants: Steffen Harzsch & Andy Sombke, Zoological Institute & Museum
Supervisors: Prof. Dr. Steffen Harzsch & Dr. Jakob Krieger, Cytology and Evolutionary Biology;
Dr. G. Torres & Dr. L. Giménez, Alfred-Wegener-Institut, Helmholtz-Zentrum für Polar- und Meeresforschung
Thesis topic: Global ocean change drives range expansion of marine species: exploring northern distribution limits
Background: Coastal and shelf seas are characterized by a high biodiversity and unique ecosystems and are fundamentally important for sustaining and enhancing human welfare and industry. It is forecasted that in the next 50 – 100 years, between 50 and 70% of the population will be living in coastal areas which creates competing demands from different sectors such as tourism, fisheries and aquaculture, on scarce coastal and shelf sea resources. Furthermore, anthropogenic stressors related to climate change already have a major impact on coastal ecosystems by affecting environmental drivers such as water temperature, salinity, pH and oxygen content. Crab species are key organisms in many marine habitats and are renowned for their massive impact in coastal ecosystems where they act as strong predators but also serve as an important link in the food web because of their complex life cycle. In many crustaceans, dispersing pelagic larvae are essential for population persistence and dispersal, and larval stages of crabs are known to be more vulnerable to fluctuations in environmental key parameters in the water column than the adult animals. Therefore, one area of research on population persistence and range expansion in crustaceans addresses the capacity of crustacean larvae to tolerate the combined effects of environmental drivers. Within the framework of RESPONSE, we have previously quantified the combined effects of abiotic stress on various aspects of larval quality in the European shore crab Carcinus maenas, an iconic member of intertidal ecosystems of many European coasts. These experimental studies were carried out at the Alfred-Wegener-Institute (AWI) at the island of Helgoland using animals from the local population of Carcinus maenas. Because multi-population comparisons are instrumental to species responses to climate change, we are also analysing if larvae of this species taken from mothers from different populations across most of its native range (from S. Spain to S. Norway) display different levels of resistance to environmental stress (temperature and salinity).
Goals of the project: Our previous studies have suggested, C. maenas has a strong potential to expand its native range northwards along the Norwegian coast, as a consequence of global warming. The project advertised here seeks to predict the future northern range expansion of this species and will be carried out in collaboration with Dr. Gabriela Torres (AWI Helgoland) and Dr. Luis Giménez (AWI Helgoland and School of Ocean Sciences, Bangor University, Wales). In a common garden experiment, larvae from Norwegian crab populations will be reared under a previously tested regime of thermal stress at the AWI Helgoland. To that end, we will compare the performance of larvae from edge populations at the northern distribution limit with those from the core of the Norwegian distribution range. Building on the expertise from the previous project, we will measure key parameters of larval performance such as survival, developmental duration, respiration and feeding rates, biomass, and gene expression of target genes. In a second step, we will establish predictions for the northern range expansion of this species using phenological models.
Requirements: Candidates should have a strong background in aquatic biology (e.g. ecology or physiology), must be prepared to work independently for extended time periods at a marine biological station and should have experience in culturing marine organisms. The candidate should also be prepared to carry out field sampling over extended time periods and spatial scales (including having a driving licence). It is desired that candidates have good knowledge of statistical methods and some basic knowledge of programming in R or a similar software (e.g. Matlab, Python). These skills will facilitate the development of modelling approaches for species persistence predictions. Organizational skills and ability to work in a team are also essential as well as a profound knowledge of the English language (speaking and writing) as well as basic knowledge in German.
Associated scientists: Zoran Šargač, Jakob Krieger & Steffen Harzsch, Zoological Institute & Museum
Research question: This project complements RESPONSE by quantifying the combined effects of abiotic drivers (temperature and salinity as proxy for expected ocean change conditions) on various aspects of larval quality as a key feature for crustacean’s potential for dispersal in a multi-population common garden approach.
State of the art: Decapod crustaceans are key species in many marine habitats, and crab species are renown for their massive impact in coastal ecosystems (Environ Sci Pollut Res 21: 9129). Crabs display a complex life cycle that include pelagic larvae in addition to the benthic juvenile-adult stages (Invertebr Reprod Dev 49: 175). Crab larvae of the Zoea type, as the main dispersing stage, are essential for range expansion and population persistence and connectivity (Biol Rev 125: 3465) and represent the life history stage that is most sensitive to fluctuations of environmental parameters. Global ocean change is already affecting abiotic factors such as temperature, salinity and pCO2 (Nat Clim Change 6: 83). In particular, semi-enclosed seas such as the Baltic Sea and North Sea will be increasingly affected by rising surface temperatures and decreasing salinities due to a higher river run-off (Global Change Biol 21: 117). Therefore, research on the impacts of global ocean change on marine organisms focusses on possible synergistic and antagonistic effects of multiple environmental drivers (Global Change Biol 24: 2239, Ecol Lett 21: 568). Because for a given population, one way of reacting to environmental changes is shifting its range, in the north European seas, climate models predict that various taxa will extend their range northwards, and these models coincide with current observations (Nat Clim Change 6: 83, Evol Applicat 7: 104). Species which are not able to track their preferred environment in space may adapt in situ to avoid extinction when the rate of environmental change is high, and these new selective pressures may lead to the evolution of new genetic adaptions or increased phenotypic plasticity (Evol Applic 7: 104). The larvae of decapod crustaceans are well-established models in Ecological Developmental Biology, a discipline that seeks to examine how organisms develop in “real-world” environments including insights into the organism’s evolutionary potential to adapt to the changing physical and biotic environmental conditions created by anthropogenic climate change. Because survival of early life stages of crustaceans will determine patterns of recruitment and population persistence, research on future species distribution in crustaceans addresses the capacity of larvae to tolerate e. g. thermal and osmotic stress (Scientif Rep 6: 32263, Mar Biol 150:1275, Aquat Biol 12: 249).
Previous work: The senior PI published a first paper on larval development of the European shore crab Carcinus maenas some 25 years ago1. Our group now studies the evolution of arthropod nervous systems 1-4 including C. maenas5 and aspects of crustacean development 6, 7. Here, we have quantified the combined effects (reaction norms) of multiple drivers on larval development of C. maenas in an experimental array covering optimal and sub-optimal conditions (factorial design: 3 salinities x 4 temperatures). Larval rearing was done at the Alfred-Wegener-Institute Helgoland (Helmholtz Centre for Polar & Mar. Res.). As proxies for larval quality, we measured developmental performance (survival rate, developmental duration), morphological (geometric morphometry) and anatomical traits (microCT, histology, 3D reconstruction), and biochemical composition (dry weight, elemental composition, lipid, protein) as well as metabolic enzymes (pyruvate kinase and citrate synthase). Based on microCT scans8, we first established a 3D model and histological atlas of larval organogenesis at optimal rearing conditions as the basis for the analysis of organogenesis during the stress treatments9. As for developmental performance, all measured parameters showed that synergistic effects of dual stressors exert stronger effects on larval quality than single stressors, thus highlighting the importance of multiple driver experiments in analyses that may contribute to predictions of global ocean change.
Working hypotheses and work plan: For marine ecosystems, multi-population comparisons using common-garden approaches (Heredity 116: 249) are more and more seen as instrumental to understand local adaptation and phenotypic plasticity in order to predict pending ecosystem changes (Scientif Rep 6: 32263, Mar Biol 150:1275). Therefore, we will assess if C. maenas larvae from mothers from different populations across its native range display different levels of resistance to environmental drivers (first PhD topic) to contribute insights that help us predicting range expansions of C. maenas in the future ocean (second PhD topic). Within northern Europe, the North Sea with its temperature gradient from the Spain into Norway and a salinity gradient across Skagerak and Kattegat into the Baltic Sea provides an ideal natural playground to analyse phenotypic plasticity versus genetic adaptations to changing environmental parameters at the level of local populations of a single species (Evol Applic 7: 104). Quantifying larval reaction norms is important because temperature controls the dispersal potential through changes in the length of the dispersal phase and through effects on larval fitness. Exposure to low salinity can lead to either increased mortality or reduced growth in dispersive larval stages (Aquatic Biol 12: 249-260). We will analyse larvae from females obtained from different local populations of C. maenas along a temperature and a salinity gradient: Norway vs. Portugal, Wales vs. Helgoland vs. Baltic. We will rear larvae of selected populations (e.g. Norway – Portugal for temperature gradient; Helgoland – Baltic for salinity gradient) under the previously tested (phase 1) regime of combined abiotic thermal and osmotic stress. As in the previous project, we will measure larval survival, developmental duration, dry weight, elemental composition. We will also measure a previously identified morphological key trait, the amount of lipid inclusions in resorptive cells (R cells) of the digestive epithelium in the midgut gland as the central metabolic organ. As additional morphological traits, we will perform immunolocalization of NaKATPase in the larval transport epithelia. For larvae obtained from all populations (extract larval rRNA on site), we will perform molecular analyses by real time qPCR to measure NaKATPase (NKA), NaClCotransporter, and heat shock proteins using established primers for these enzymes in adult C. maenas. We will also quantify the NKA protein expression using Western Blot analysis. From all populations, we will obtain samples for population genetic studies done by our project partners. Species distribution predictions of C. maenas so far have been based on thermogeography, heat tolerance and potential for thermal acclimation of adult animals (J Exp Biol 217: 1129). Using modelling approaches, together with the phase 3 PostDoc, we will use information on larval reaction norms in combination with available data on thermogeography of adults to contribute to predictions about the range expansion of this species in the future ocean.
Envisaged thesis topics: 1) Development of crab larvae Carcinus maenas from different European populations reared in combined thermal and osmotic stress; 2) Towards predicting the global range of C. maenas in the future ocean.
Associated scientists: Franziska Spitzner, Andy Sombke & Steffen Harzsch
State of the art: Climate change already has a major impact on marine ecosystems including plankton communities (Prog. Oceanograph. 81: 207). In particular semi-enclosed seas (e.g. the Baltic Sea) will be increasingly affected by rising surface temperatures and decreasing salinity due to a higher river runoff (J. Exp. Mar. Biol. Ecol. 400: 52). Decapod crustaceans may be particularly sensitive to such changes, owing to their complex life cycle including a pelagic larval and a benthic juvenile-adult stage. Here, larval survival critically depends on the ability to respond appropriately to chemical, mechanosensory and visual cues to control tidal transport and onshore recruitment (Invert. Reprod. Dev. 49: 175). Larval settlement is of utmost importance for colonisation and population survival, relying on e.g. odour identification indicating preferred habitats and the presence of conspecifics (Mar. Freshwater Behav. Physiol. 39: 269). Hence, the ability to sense environmental cues is an ecologically important trait intimately associated with fitness. However, larval growth and bioenergetics in decapod larvae are strongly affected by thermal and osmotic stress (Invert. Reprod. Dev. 33: 159). While concomitant effects on growth patterns are well understood, knowledge on the effects of abiotic stress on the development of specific, energy-demanding organs such as the nervous system and hence on behavioural performance is lacking. Therefore, we will here explore the effect of abiotic stress on the development of the larval olfactory system and olfactory-guided behaviour.
Working hypotheses and work plan: Current climate change may expose crustacean planktonic larvae to increasing abiotic stress (see above). We hypothesise that (1) thermal and salinity stress will negatively affect neurogenesis and thereby larval behavioural performance, and that (2) negative effects will differ depending on the respective species’ ability to tolerate challenging environmental conditions. To this end, we will study the effects on osmotic and thermal stress on the development of the olfactory system in larvae of six European decapod crustaceans, which are known to differ in their stress tolerance (Aquat. Biol. 21: 249). Investigations will include (1) in vivo incorporation of an s-phase specific mitosis marker to quantify neurogenesis; (2) immunohistochemistry against neuropeptides and confocal laser-scan microscopy to analyse the formation of neuronal networks within the central olfactory pathway, (3) scanning and transmission electron microscopy to analyse the ontogeny of the chemosensory organs; (4) bioessays to analyse larval responses and sensitivity ranges to environmental stimuli. A major part of the breeding experiments will be carried out at the “Biologische Anstalt Helgoland”, a marine biological station on the island of Helgoland/North Sea (http://www.awi.de/de/institut/standorte/helgoland/) in cooperation with Dr. Gabriela Torres and Dr. Luis Gimenez (Bangor, Wales).
Thesis topic: Effects of abiotic stress on neurogenesis in the developing brain of crustacean larvae.
DFG-funded project
Applicant: Prof. Dr. Steffen Harzsch (University of Greifswald)
The current project sets out to explore differences and similarities in the developmental processes of insect and crustacean olfactory systems. Considering insects are seen as a diverging crustacean lineage which very successfully invaded terrestrial habitats our proposed research is driven by the question if, from a crustacean ground pattern, holometabolous insects (from which most of our knowledge stems) have evolved alternative solutions for making olfactory systems than crustaceans. The goal of this application is to establish the crustacean ground pattern with a focus on the emergence of the olfactory sensory neurons and the formation of the glomeruli, anew data set against which the existing insect data can then be compared. We expect developmental differences between insect and crustacean olfactory systems and therefore ask the following questions:
- The general life cycles of holometabolous insects and malacostracan crustaceans and their modes of neuronal development which in the Holometabola is based on imaginals discs show distinct differences (Chipman 2015, Jockusch and Smith 2015, Scholtz 2020). How do these fundamental differences effect formation of the olfactory system?
- Crustacean antennae grow throughout life and new aesthetascs with olfactory sensory neurons (OSNs) are added at each molt, continuously increasing the sensory input to the central olfactory system. In addition to growth, there is a continual turnover and renewal of the sensory receptor system during adult life but such a long growth phase and dynamic turnover and renewal is unknown in insects. What are the dynamics of embryonic and larval OSN proliferation in the three crustaceans target taxa?
- Crustaceans most likely do not display a 1:1 wiring logic from receptor molecule to glomerulus. During their evolutionary diversification from the crustaceans, insects optimized their olfactory systems by tuning the connectivity from olfactory sensory receptor to the olfactory glomeruli towards the 1:1 wiring logic that we find today e.g. in the fruit fly. Are these principle differences mirrored in the process of formation and stabilization of the glomeruli, specifically, the dynamics of axon ingrowth and their interaction with glia cells and the local interneurons?
- Malacostracan crustaceans seem to possess two to three orders of magnitude more interneurons in their central olfactory pathway than insects suggesting that insects have evolved mechanisms to economize on the number of neuronal elements in their central olfactory pathway and shortened the period of cell proliferation. What differences do we find in the dynamics and cellular mechanisms of embryonic and larval cell proliferation in insects versus crustaceans?
- Crustaceans typically have higher glomerular numbers than insects and to date we do not know of any individually identifiable glomeruli in crustaceans, opposite to insects which economized on the number of olfactory glomeruli by assigning individual identities to these elements. During crustacean development, do we find identifiable glomeruli at some stage?
Contrary to many derived Malacostraca, the glomeruli of pterygote insects are mostly spherical and not commonly arranged radially around the periphery of the central non-synaptic fiber core. Insects display much more plasticity in the geometrical arrangement of their glomeruli than crustaceans, suggesting that different constructional constraints may apply. Does studying glomerular formation provide insights into such constructional constraints?
DFG-funded project
Applicant: Prof. Dr. Steffen Harzsch (University of Greifswald), Dr. Jürgen Rybak (MPI Chemical Ecology, Jena), Prof. Dr. Martin Nawrot (University of Cologne)
Thematic focus 1: The evolution of core circuits in PP 2205 „Evolutionary Optimisation of Neuronal Processing“
The main chemosensory organ of insects and crustaceans for distance olfaction is the most anterior pair of head appendages, the deutocerebral antennae. Olfactory sensory neurons associated with sensilla on the antennae project their axons into the brain’s primary olfactory centres, the bilaterally paired antennal lobes (insects)/olfactory lobes (crustaceans). There, sensory olfactory afferents synapse with two classes of olfactory interneurons (OSNs), the local olfactory interneurons (LNs) and the olfactory projection neurons (PNs), within specialized neuropil compartments, the olfactory glomeruli. Although the principle wiring pattern within the glomeruli of crustaceans and insects share many similarities, there also exist pronounced difference of these olfactory core circuits. Therefore, this system can be seen as an ideal playground for those interested in analysing evolutionary diversification of neuronal core circuits. By comparing insects and crustaceans we can analyse the end point of past evolutionary optimization processes by analyzing which potential alternative solutions arthropods have found for olfactory circuits. This project brings together two experts on the functional and evolutionary morphology of crustacean (HAR) and insect (RYB) olfactory system and a computational neuroscientist with a strong background in arthropod olfactory systems (NAW). We want to gain detailed insights into the neuroanatomy of the central olfactory pathway beyond the well studied model system D. melanogaster. By quantifying the numbers of the involved neuronal elements (olfactory sensory neurons, local olfactory interneurons, projection neurons, olfactory glomeruli) and by analysing architectural characteristics such as volume, shape and geometrical properties of olfactory glomeruli we will gain additional insights into divergence versus convergence and evolutionary specialization of the central olfactory pathways in arthropod lineages with a known evolutionary history. Furthermore, by obtaining connectome-level data we will understand the evolutionary optimization of local circuit motifs, specifically the connectivity of OSNs to PNs. Finally, by developing a mathematical model of information processing within olfactory glomeruli of different arthropod lineages, we want to understand possible differences of olfactory coding mechanisms in insects versus crustaceans.
DFG-funded project
Applicant: Dr. Georg Brenneis
Sea spiders (Pycnogonida) are a marine chelicerate taxon that is the likely sister group of Euchelicerata (comprising horseshoe crabs, scorpions, spiders and their kin). Due to this interesting phylogenetic position, the study of pycnogonids holds the potential to inform on organ system transformations during early chelicerate and - even more generally - arthropod evolution.
This project aims to (1) compare adult neuroanatomy across the major taxa (“families”) of sea spiders and (2) study adult neurogenesis in one of their representatives. Extant pycnogonids show an astoundingly uniform body organization, featuring the “head” region with three specialized appendage pairs and the trunk with four walking leg pairs. However, while walking leg structure is strictly conserved, the “head” appendages show remarkable taxon-specific structural variations. Yet, neither these external morphological characteristics, nor molecular sequence data have so far enabled robust reconstruction of pycnogonid phylogeny, rendering scrutiny of internal anatomy overdue. In this context, the central nervous system (CNS) is a promising candidate organ to explore, as the usefulness of neural characters for phylogenetic inference has been previously shown for the major arthropod lineages.
Using a range of modern-day techniques, CNS regions will be 3D-reconstructed to provide a supracellular backbone for high-resolution studies at the single cell level via immunolabeling of various neuroactive substances. Additionally, in-vivo cell proliferation experiments coupled with gene expression studies and neuronal backfills will explore adult neurogenic processes – an understudied phenomenon that may underlie plasticity of specific CNS areas in many arthropod taxa.
The results will be the basis for the first data matrix on an internal organ system of Pycnogonida. Cladistic analyses will assess the resolution power of neural characters for pycnogonid phylogeny, acting as a case study for evolutionarily old arthropod taxa with limited morphological disparity. Further, the analyses will enable critical evaluation of the available unstable and incongruent morphological and molecular hypotheses on sea spider phylogeny. Neuroanatomical features of the pycnogonid ground pattern will be reconstructed and compared to other arthropod lineages, which will contribute to our understanding of chelicerate and arthropod nervous system evolution. Among others, it is projected to reveal additional apomorphies of the morphologically still unsatisfactorily supported monophylum (Pycnogonida + Euchelicerata).
DFG-funded project
Applicant: Dr. Andy Sombke, PD Dr. Carsten H.G. Müller
Associate Researcher: Dr. Matthes Kenning
The arthropodium has adapted many different shapes and is thus able to perform a vast of different functions. Among arthropod taxa, trunk legs, primarily used for locomotion, were transformed many times independently into appendages of various functions and are thus considered excellent study models to understand pathways of adaptation and evolution. The aim of this project is to investigate the terminal legs in centipedes, which do not participate in walking. Often correlated with sexual dimorphism, terminal legs may be extremely elongated and resemble antennae. We explore the sensory equipment, innervation patterns and function of terminal legs of selected centipede species using multimodal microscopic (SEM, TEM, LM, cLSM) and electrophysiological techniques. Comparisons with antennae and walking legs will unravel potential constructional morphological constraints in the course of evolutionary transformation from former walking legs in what is assumed to be sensory appendages.

DFG-funded project
Applicant: Prof. Dr. Steffen Harzsch (Greifswald)
Cooperation Partner: Prof. Dr. Barbara S. Beltz (Wellesley)
The central olfactory pathway of the crayfish brain is characterized by persistent, life-long neurogenesis. Adult neurogenesis is driven by a neurogenetic niche on the surface of the brain that generates 1st generation precursor cells. These cells leave the niche and move along an anteriorly and a medially directed migratory stream towards an anterior and a medial proliferation zone, both associated with the central olfactory pathway. During their migration, the cells undergo additional mitoses and evolve into 2nd and 3rd generation precursor cells. The medial proliferation zone contributes new local olfactory interneurons whereas the lateral proliferation zone generates olfactory projection neurons. The cellular machinery in this system that is collectively called the “deutocerebral proliferative system” (DPS) displays strong similarities to the system that drives adult neurogenesis in mammals. In these animals, a neurogenetic niche, the subventricular zone, generates progeny that migrate along the rostral migratory streams towards the olfactory bulb where the neurons are integrated into existing neuronal circuits. This projects sets out to examine the phenomenon of persistent neurogenesis in the crustacean olfactory system in a broad comparative context and against an evolutionary background. We want to know if the mechanisms of adult neurogenesis described for the crayfish hold for other malacostracan crustaceans too and will analyse possible variations in order to gain insights into the possible function of this system. With this comparative approach we will follow an evolutionary experiment and explore the solutions which crustaceans evolved for the task „life-long neurogenesis“. To that end we will study ca. 10 – 15 representative of all major taxa of Malacostraca with immunohistochemical techniques, in vivo labelling with combinations of mitosis markers, pulse-chase experiments and transmission electron microscopy. Specifically, we will analyze A) general arrangement of the DPS components; B) ultrastructure of the neurogenetic niche; C) dynamics of the directed movement in the migratory streams; D) integration of the new olfactory interneurons into the central olfactory pathway; E) persistent neurogenesis associated with the hemiellipsoid bodies in the lateral protocerebrum. This study will lay the foundations for a future project that will in more depth explore the function of persistent neurogenesis in suited model crustaceans specifically with regard to the question how the new neurons are wired up into the network of the functional, adult olfactory system.
DFG-funded project
Applicants: Prof. Dr. Steffen Harzsch (Greifswald), Prof. Dr. Bill S. Hansson (Jena)
In parallel to insects, terrestrial Crustacea provide a fascinating chance to participate in a wonderful evolutionary experiment by analyzing which potential alternative solutions arthropods have evolved to explore the terrestrial olfactory landscape. General questions of our approach include:
- Have terrestrial Crustacea successfully established aerial olfaction at all, and which subgroups?
- How have they solved the task of detecting airborne stimuli at the level of olfactory sensory neurons and their receptors?
- Have they evolved insect-like odorant binding proteins and agents with anti-fouling functions to fight epibionts?
- How have the dramatic changes in selection pressure on the sensory systems reshaped and modified peripheral and central olfactory pathways?
- Does their antenna display ultrastructural specializations that may play a role for odorants to get in contact with olfactory sensory neurons?
- To what odor components do they respond and are the response profiles different from those of insects?
- Which tracking strategies do they use to locate odor sources and do these resemble those of walking or flying insect odor trackers?
- Have they evolved insect-like behaviors to track odor sources and which role does air flow play in their tracking behavior?
- Which mechanosensors do they use to detect flow?
- Which role do differences in sensor spans play in terrestrial crustaceans of different sizes?
The chemical sense of marine isopod crustaceans (DFG HA 2540/9-1)
DFG-funded project
Applicant: Prof. Dr. Steffen Harzsch, Dipl. Biol. Matthes Kenning
Cooperation Partner: Dr. Magnus Lindström (University of Helsinki)
Our current knowledge on both the morphology of the crustacean olfactory pathway and on chemically guided behavior is heavily biased towards members of the Decapoda. In the current project, we want to gain more insights into olfaction in another major group of malacostracan crustaceans, the marine Isopoda by analysing the architecture of the peripheral and central olfactory pathway and by conducting behavioral essays. To that end we propose here to analyze, in a wide range of isopods, the morphology of the antennae and the central olfactory pathway with neuroanatomical methods such as scanning electron microscopy, serial semithin sectioning combined with 3D reconstruction, antennal backfilling with neuronal tracers, focal application of dextrans to label populations of olfactory interneurons, and immunofluorescence combined with confocal laser-scan microscopy. We will try to analyse representatives from a wide range of different habitats including the deep sea, the Arctic and Antartica, the Tropics, and from different life styles including scavengers and parasitic species. Furthermore, olfactory-guided behavior and navigation strategies to odor sources will be tested in behavioral assays using the omnivorous isopod Saduria entomon which is common in the Baltic as a model. In a neurophylogenetic approach, our data will be compared to the morphology of the olfactory systems in other malacostracan crustaceans and hexapods in order to extract data for understanding the evolution of arthropod olfactory systems in general.
DFG-funded project
Applicant: Prof. Dr. Steffen Harzsch, Dr. Andy Sombke
Cooperation Partner: Prof. Dr. Bill S. Hansson (MPI Jena)
Myriapods represent an arthropod lineage that, originating from a marine arthropod ancestor, most likely conquered land independently from hexapods. The successful transition from marine to terrestrial life requires a number of physiological adaptations that are important for survival out of water. The sensory organs of terrestrial species must be able to function in air rather than in water. In chemoreception, establishing aerial olfaction means that molecules need to be detected in gas phase instead of in water solution. In general, the neuroethology of myriapods and the architecture of their central nervous systems are poorly understood. In a set of preliminary experiments with the centipede Scutigera coleoptrata we analyzed the morphology of the antennae and the central olfactory pathway with scanning electron microscopy, serial semithin sectioning combined with 3D reconstruction, antennal backfilling with neuronal tracers, and immunofluorescence combined with confocal laser-scan microscopy. Furthermore, the ability of this animal to respond to airborne stimuli was tested in behavioral assays and electroantennogram recordings. These experiments collectively indicate a good sense of aerial olfaction in this species. Furthermore, the architecture of its olfactory neuropils is clearly distinct from hexapods and also from terrestrial crustaceans indicating an independent evolution of its olfactory sense in response to the conquest of land. We propose here to study the morphology of the central olfactory pathway in a broad range of other myriapods covering Chilopoda, Progoneata, Symphyla and combine these studies with behavioral essays. We will also include Scorpions as an outgroup. In a neurophylogenetic approach, our data will also be compared to the morphology of the olfactory systems in hexapods and crustaceans in order to extract data for reconstructing arthropod phylogeny and for understanding the evolution of arthropod olfactory systems.