I use a broad range of techniques to study the life history, social system, host-parasite interactions and population dynamics of European bat species.
Like nearly all taxa, European bat populations currently face a daunting diversity of anthropogenic threats (including habitat fragmentation, reductions in roost availability and prey density, expansion of wind energy, climate change). Given their slow life history, even small increases in mortality rate can dramatically impact populations. Therefore, the need for accurate estimates of population dynamics and a fundamental understanding of how these effects differ between species has never been more pressing. However, due to their small size, their ability to fly, and their often cryptic nature, observing and monitoring bat populations requires creative solutions.
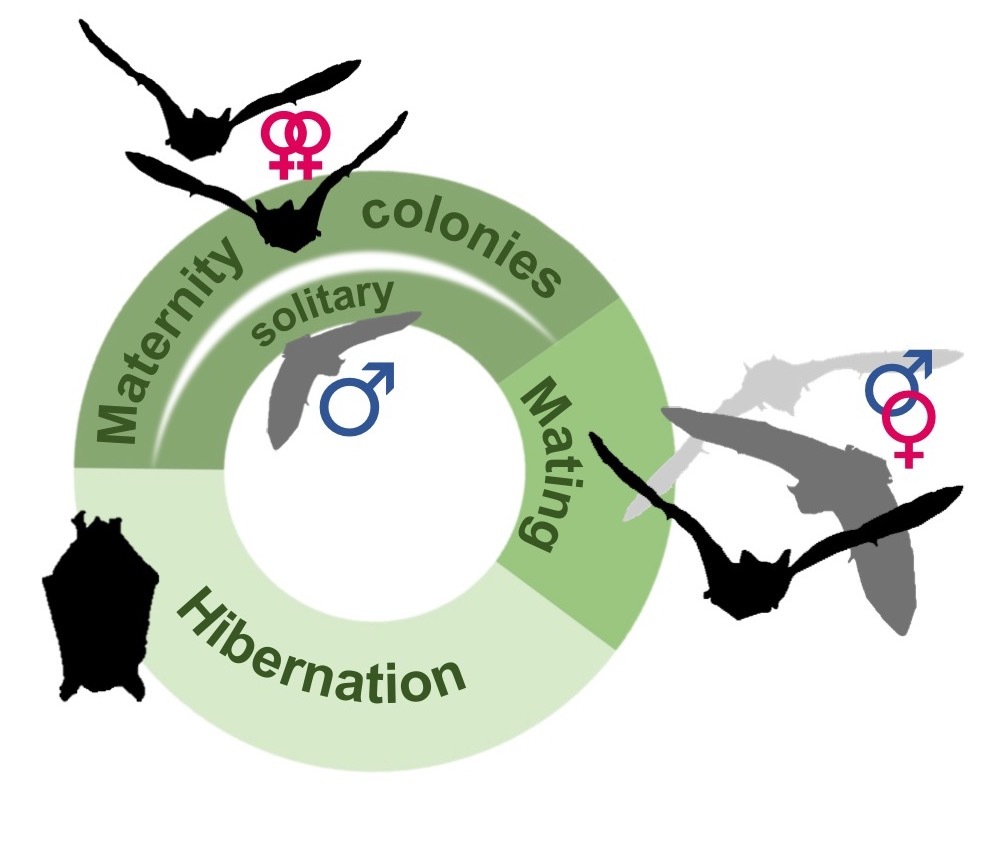
In this context, European bats offer an ideal comparative model system to explore the effects that social system has on life history and the potential consequences of environmental change. Nearly all temperate zone bat species follow a three-phase annual cycle. Female bats form summer maternity colonies. Male bats are often solitary, or form separate bachelor groups during this time. In autumn, mating takes place, either in temporary harems where males attract females to roosts that they defend, or at underground hibernacula in a promiscuous, lek-like mating system, known as swarming. During winter, individuals hibernate, either solitarily or in clusters. While all species broadly follow this cycle, their social systems nevertheless differ considerably. For example, maternity colony size may range from several, to several thousand individuals depending on the species. Likewise, species differ markedly in the degree to which individuals disperse, or interact with conspecifics from other colonies.
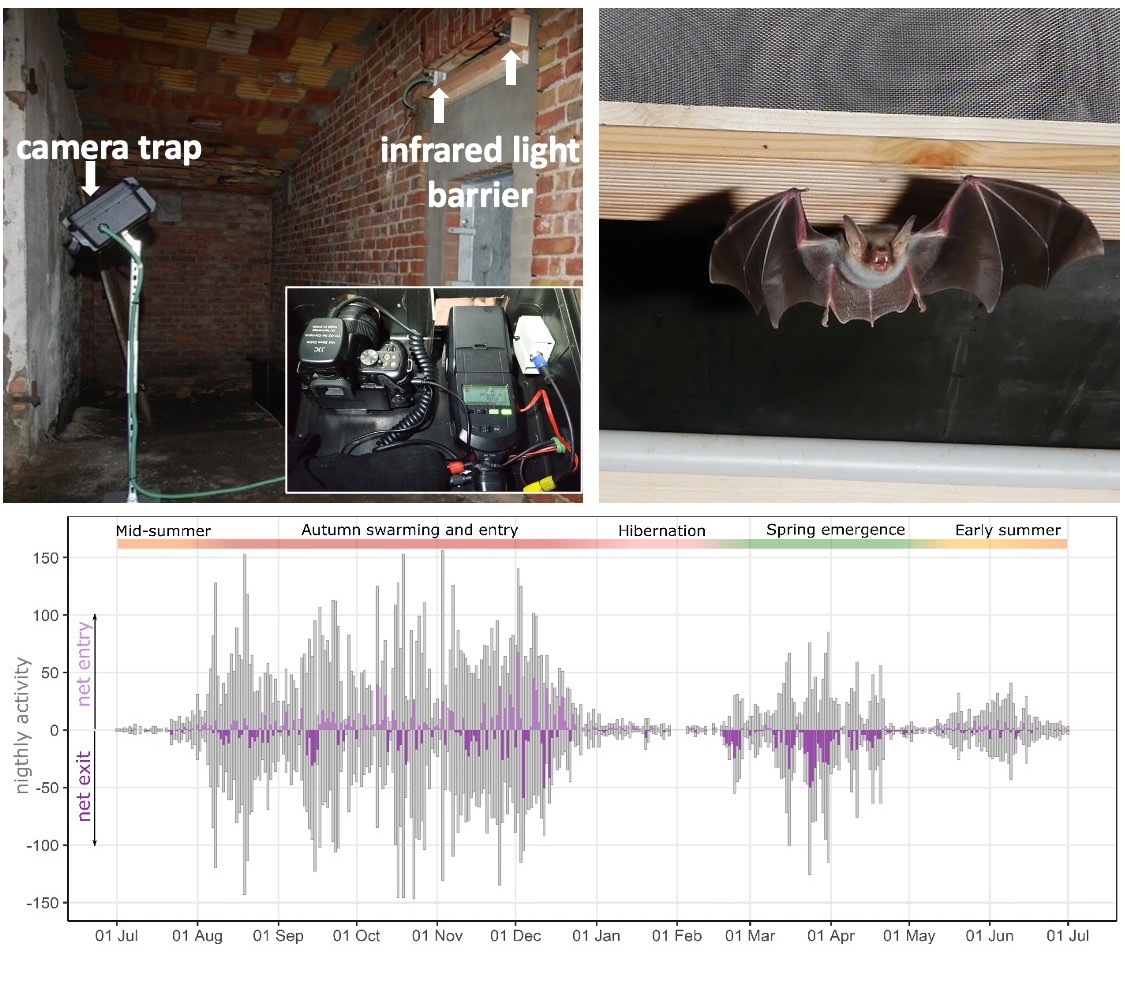
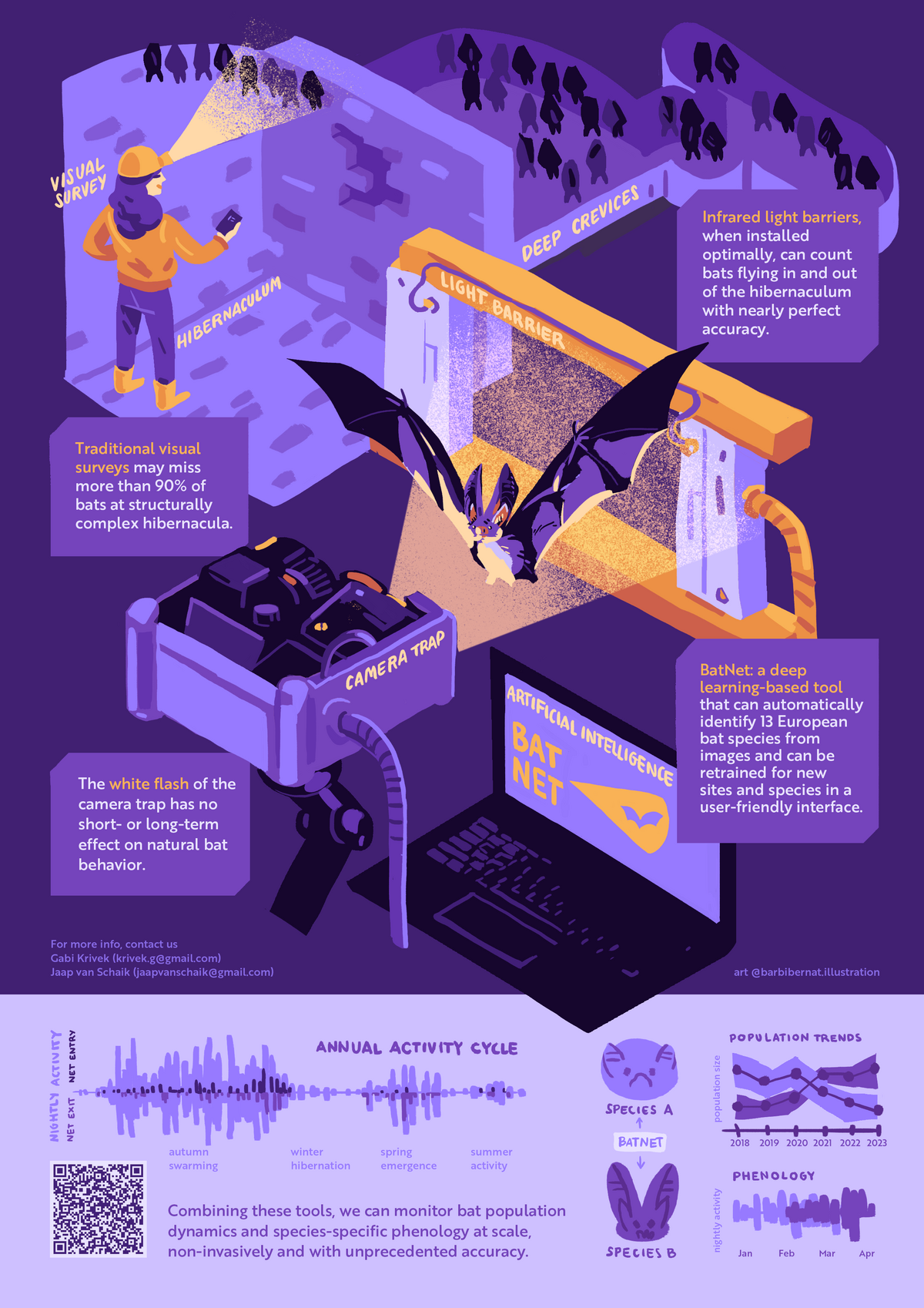
Light barriers and camera traps
Light barriers coupled with camera traps installed at the entrance to a hibernation sites, offer a unique non-invasive methodology to quantify all movements in and out of a site at unprecedented accuracy, resolution and scale. Together with Gabi Krivek, I am developing analysis tools around this method, including standardized analysis pipelines and a deep-learning solution for automated species identification (BatNet). With these we can monitor population dynamics at underground sites, and characterize the environmental drivers of hibernation phenology in different bat species.
Selected publications:
Krivek G, Gillert A, Harder M, Fritze M, Frankowski K, Timm L, Meyer-Olbersleben L, von Lukas UF, Kerth G, van Schaik J (2023). BatNet: a deep learning-based tool for automated bat species identification from camera trap images. Remote Sensing in Ecology and Conservation http://doi.org/10.1002/rse2.339
Krivek G, Mahecha EPN, Meier F, Kerth G, & van Schaik J (2023). Counting in the dark: estimating population size and trends of bat assemblages at hibernacula using infrared light barriers. Animal Conservationhttp://doi.org/10.1111/acv.12856
Krivek G, Schulze B, Poloskei PZ, Frankowski K, Mathgen X, Douwes A, & van Schaik J (2022). Camera traps with white flash are a minimally invasive method for long‐term bat monitoring. Remote Sensing in Ecology and Conservation 8(3), 284-296 https://doi.org/10.1002/rse2.243
Movement ecology and population (genetic) structure
The behavior and movement ecology of bats, and particularly the timing of the transitions between the three phases of the annual cycle remain poorly characterized. To investigate these aspects, we use a wide variety of techniques, including time-series captures and counts at underground sites, radio telemetry, RFID-tags and loggers, and genetic sampling. Current questions include how juvenile bats find suitable hibernation sites, the degree of site fidelity of bats at hibernation sites (both together with Frauke Meier and Prof. Gerald Kerth), as well as quantifying the dispersal rate and population genetic structure of several bat species including Bechstein’s bat (with Prof. Gerald Kerth), lesser horseshoe bat (with Thomas Näf and Antonia Hammer), barbastelle bat (with Moritz Krämer), and Nathusius’s pipistrelle.
Selected publications:
Reusch C, Scheuerlein A, Grosche L, Meier F, Gampe J, Dammhahn M, van Schaik J & Kerth G (2023). The risk faced by the early bat: individual plasticity and mortality costs of the timing of spring departure after hibernation. Oikos e09654 http://doi.org/10.1111/oik.09654
Meier F, Grosche L, Reusch C, Runkel V, van Schaik J§, & Kerth G§ (2022). Long-term individualized monitoring of sympatric bat species reveals distinct species-and demographic differences in hibernation phenology. BMC Ecology and Evolution22(7), 1-12 https://doi.org/10.1186/s12862-022-01962-6
Stumpf M, Meier F, Grosche L, Halczok T, van Schaik J, Kerth G (2017) Do young bats follow their mothers to swarming sites? Results from a genetic maternity assignments of two bat species at a large hibernaculum in Germany. Acta Chiropterologica 19, 319-327 https://doi.org/10.3161/15081109ACC2017.19.2.008
Dekeukeleire D, Janssen R, Haarsma A-J, Bosch T, van Schaik J (2016) Swarming Behaviour, Catchment Area and Seasonal Movement Patterns of the Bechstein's Bats: Implications for Conservation. Acta Chiropterologica 18, 349-358 https://doi.org/10.3161/15081109acc2016.18.2.004
van Schaik J, Janssen R, Bosch T, Haarsma A-J, Dekker JJA, Kranstauber B (2015) Bats swarm where they hibernate: compositional similarity between autumn swarming and winter hibernation assemblages at five underground sites. PloS one 10.7 e0130850 https://doi.org/10.1371/journal.pone.0130850
Kerth G, van Schaik J (2012) Causes and consequences of living in closed societies: lessons from a long-term socio-genetic study on Bechstein's bats. Molecular Ecology 21, 633-646 https://doi.org/10.1111/j.1365-294X.2011.05233.x
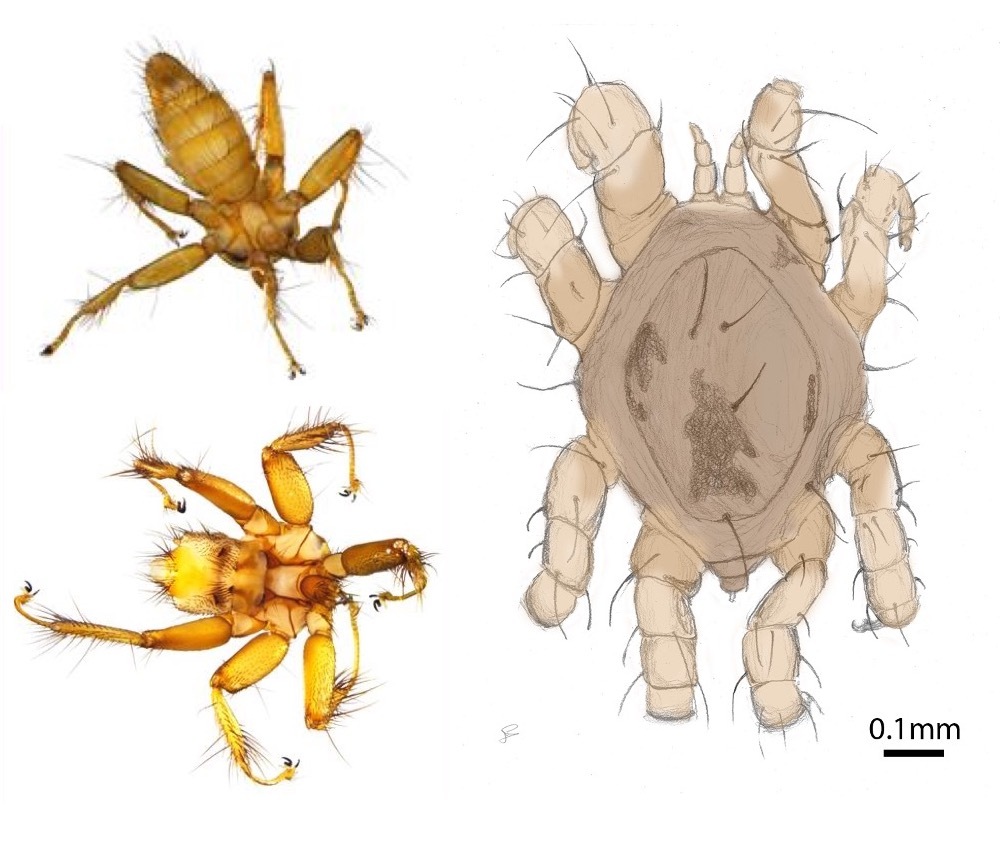
Host-parasite evolutionary dynamics
In addition to the bats themselves, the differences in mating system and social organization seen in European bats offer an ideal comparative framework to explore how such differences in host sociality affect the transmission and genetic structure of their obligate ectoparasites and pathogens. I use comparative population genetics and time series sampling of ectoparasites from wild bat populations to investigate how variation in social system interact with parasite life-history characteristics to shape the transmission dynamics, and the broad- and local-scale population genetic structure of the parasites. This work focuses primarily on Spinturnicid wing-mites and Nycteribid bat flies, as well as a pathogen that is vectored by the bat flies: haemosporidians of the genus Polychromophilus (Together with Dr. Juliane Schaer, Humboldt University Berlin and Branka Bajíc, University of Belgrade, Serbia). In addition, I am using similar techniques to investigate the prevalence and genetic diversity of tick-borne pathogens in Sifakas together with Dr. Rebecca Lewis (University of Texas-Austin).
Selected publications:
Bajić B, Werb O, Budinski I, Blagojević J, Schaer J§, van Schaik J§ (2023) Non-invasive investigation of Polychromophilus parasite infections in bat populations in Serbia using bat flies. Parasites & Vectors
Pejić B, Budinski I, van Schaik J§, & Blagojević J§ (2022). Sharing roosts but not ectoparasites: high host-specificity in bat flies and wing mites of Miniopterus schreibersii and Rhinolophus ferrumequinum (Mammalia: Chiroptera). Current Zoology 68(5), 507-516 https://doi.org/10.1093/cz/zoab086
van Schaik J, Dekeukeleire D, Gazaryan S, Natradze I, Kerth G (2018) Comparative phylogeography of a vulnerable bat and its ectoparasite reveals dispersal of a non-mobile parasite among distinct evolutionarily significant units of the host. Conservation Genetics 19, 481-494 https://doi.org/10.1007/s10592-017-1024-9
van Schaik J, Kerth G (2017) Host social organization and mating system shape parasite transmission opportunities in three European bat species. Parasitology Research 116, 589-599 https://doi.org/10.1007/s00436-016-5323-8
van Schaik J, Dekeukeleire D, Kerth G (2015) Host and parasite life history interplay to yield divergent population genetic structures in two ectoparasites living on the same bat species. Molecular Ecology 24, 2324-2335 https://doi.org/10.1111/mec.13171
van Schaik J, Kerth G, Bruyndonckx N, Christe P (2014) The effect of host social system on parasite population genetic structure: comparative population genetics of two ectoparasitic mites and their bat hosts. BMC Evolutionary Biology 14, 18 https://doi.org/10.1186/1471-2148-14-18
- Gabriella Krivek: Automatic image-based species identification of European bats using deep learning algorithms (part of the DIG-IT! Landesexzellenzprogramm MV)
- Thomas Näf: Range expansion and dispersal in lesser horseshoe bats (GRK 2010 Biological RESPONSEs to Novel and Changing Environments), 2nd Cohort
- Antonia Hammer: Range expansion and dispersal in lesser horseshoe bats (GRK 2010 Biological RESPONSEs to Novel and Changing Environments), 3rd Cohort
- Frauke Meier: Localization and use of hibernacula in temperate zone bats
- Moritz Krämer: Population genetic structure and range expansion of the Barbastelle bat (Barbastella barbastellus) in Germany (Part of the Verbundprojekt: “Schutz und Förderung der Mopsfledermaus in Deutschland“, https://www.mopsfledermaus.de )
Samuel Schuler (MSc): title TBA
Benjamin Dötterl (MSc): title TBA
Former students: Luisa Timm (MSc), Karina Frankowski (MSc), Anja Fritzsche (MSc), Laura Maaß (MSc), Lucie Hoffschläger (BSc), Daan Dekeukeleire (MSc)
MSc BEE Module E1.9 & E1.10 / MSc LENC Module E24 & E25; Every WiSe
Conservation and Landscape Genetics (Lecture)
Current topics in Conservation Genetics (Seminar)
Applied Conservation Genetics (Practical)
Google Scholar: https://scholar.google.de/citations?user=acnwIxQAAAAJ
ORCID: 0000-0003-4825-7676
§ indicates shared first/senior authorship
Meier, F., Grosche, L., Krivek, G., Runkel, V., Scheuerlein, A., Kerth, G., & van Schaik, J. (2025). Automated long‐term monitoring of RFID‐tagged individuals reveals high hibernaculum site fidelity in Daubenton's bats and Natterer's bats. Animal Conservation, 28(3), 401-409. https://doi.org/10.1111/acv.12992
van Schaik, J., Schuler, S., Stienstra, K., Janssen, R., Dekeukeleire, D., Boshamer, J.P., Noort, B.C., Steenbergen, J. and Lagerveld, S. (2025). Diverse but declining? Population genetic structure and genetic diversity of Nathusius’ pipistrelle along the Dutch coastline during the autumn migration period. Mammalian Biology, 1-12. https://doi.org/10.1007/s42991-025-00486-y
Timm, L., Rosskopf, S. P., Werb, O., van Schaik, J., & Schaer, J. (2025). Europe-wide distribution and bat-host specific lineages in the malarial parasite Polychromophilus murinus revealed through genetic screening of bat flies. Infection, Genetics and Evolution, 127, 105707. https://doi.org/10.1016/j.meegid.2024.105707
Mundinger C, van Schaik J, Scheuerlein A & Kerth G (2023). Heat over heritability: Increasing body size in response to global warming is not stabilized by genetic effects in Bechstein's bats. Global Change Biology, 00, 1– 10. https://doi.org/10.1111/gcb.16824
Bajić B, Werb O, Budinski I, Blagojević J, Schaer J§, van Schaik J§ (2023) Non-invasive investigation of Polychromophilus parasite infections in bat populations in Serbia using bat flies. Parasites & Vectors
Krivek G, Gillert A, Harder M, Fritze M, Frankowski K, Timm L, Meyer-Olbersleben L, von Lukas UF, Kerth G, van Schaik J (2023). BatNet: a deep learning-based tool for automated bat species identification from camera trap images. Remote Sensing in Ecology and Conservation http://doi.org/10.1002/rse2.339
Naef T, Besnard, A-L, Lehnen L, Petit EJ, van Schaik J§, Puechmaille SJ§ (2023). How to quantify factors degrading DNA in the environment and predict degradation for effective sampling design. Environmental DNA http://doi.org/10.1002/edn3.414
Krivek G, Mahecha EPN, Meier F, Kerth G, & van Schaik J (2023). Counting in the dark: estimating population size and trends of bat assemblages at hibernacula using infrared light barriers. Animal Conservation http://doi.org/10.1111/acv.12856
Reusch C, Scheuerlein A, Grosche L, Meier F, Gampe J, Dammhahn M, van Schaik J & Kerth G (2023). The risk faced by the early bat: individual plasticity and mortality costs of the timing of spring departure after hibernation. Oikos e09654 http://doi.org/10.1111/oik.09654
Kerth G, van Schaik J (2022). Bechstein’s Bat Myotis bechsteinii (Kuhl, 1817). In: Hackländer, K., Zachos, F.E. (eds) Handbook of the Mammals of Europe. Handbook of the Mammals of Europe. Springer, Cham. doi.org/10.1007/978-3-319-65038-8_58-1
Kappeler PM, Benhaiem S, Fichtel C, Fromhage L, Höner OP, Jennions MD, Kaiser S, Krüger O, Schneider JM, Tuni C, van Schaik J & Goymann W (2022). Sex roles and sex ratios in animals. Biological Reviews 98: 462-480 https://doi.org/10.1111/brv.12915
Meier F, Grosche L, Reusch C, Runkel V, van Schaik J§, & Kerth G§ (2022). Long-term individualized monitoring of sympatric bat species reveals distinct species-and demographic differences in hibernation phenology. BMC Ecology and Evolution22(7), 1-12 https://doi.org/10.1186/s12862-022-01962-6
Pejić B, Budinski I, van Schaik J§, & Blagojević J§ (2022). Sharing roosts but not ectoparasites: high host-specificity in bat flies and wing mites of Miniopterus schreibersii and Rhinolophus ferrumequinum (Mammalia: Chiroptera). Current Zoology 68(5), 507-516 https://doi.org/10.1093/cz/zoab086
Krivek G, Schulze B, Poloskei PZ, Frankowski K, Mathgen X, Douwes A, & van Schaik J (2022). Camera traps with white flash are a minimally invasive method for long‐term bat monitoring. Remote Sensing in Ecology and Conservation 8(3), 284-296 https://doi.org/10.1002/rse2.243
Stapelfeldt B, Schöner M, Kerth G, & van Schaik J (2020). Slight increase in bat activity after human hibernation count monitoring of a bunker complex in northern Germany. Acta Chiropterologica 22(2), 383-390 https://doi.org/10.3161/15081109acc2020.22.2.012
Gill LF, van Schaik J, von Bayern AM, & Gahr ML (2020). Genetic monogamy despite frequent extrapair copulations in “strictly monogamous” wild jackdaws. Behavioral Ecology 31, 247-260 https://doi.org/10.1093/beheco/arz185
van Schaik J, Dekeukeleire D, Gazaryan S, Natradze I, Kerth G (2018) Comparative phylogeography of a vulnerable bat and its ectoparasite reveals dispersal of a non-mobile parasite among distinct evolutionarily significant units of the host. Conservation Genetics 19, 481-494 https://doi.org/10.1007/s10592-017-1024-9
Stumpf M, Meier F, Grosche L, Halczok T, van Schaik J, Kerth G (2017) Do young bats follow their mothers to swarming sites? Results from a genetic maternity assignments of two bat species at a large hibernaculum in Germany. Acta Chiropterologica 19, 319-327 https://doi.org/10.3161/15081109ACC2017.19.2.008
van Schaik J, Kerth G (2017) Host social organization and mating system shape parasite transmission opportunities in three European bat species. Parasitology Research 116, 589-599 https://doi.org/10.1007/s00436-016-5323-8
Dekeukeleire D, Janssen R, Haarsma A-J, Bosch T, van Schaik J (2016) Swarming Behaviour, Catchment Area and Seasonal Movement Patterns of the Bechstein's Bats: Implications for Conservation. Acta Chiropterologica 18, 349-358 https://doi.org/10.3161/15081109acc2016.18.2.004
van Schaik J, Janssen R, Bosch T, Haarsma A-J, Dekker JJA, Kranstauber B (2015) Bats swarm where they hibernate: compositional similarity between autumn swarming and winter hibernation assemblages at five underground sites. PloS one 10.7 e0130850 https://doi.org/10.1371/journal.pone.0130850
van Schaik J, Dekeukeleire D, Kerth G (2015) Host and parasite life history interplay to yield divergent population genetic structures in two ectoparasites living on the same bat species. Molecular Ecology 24, 2324-2335 https://doi.org/10.1111/mec.13171
van Schaik J, Kerth G, Bruyndonckx N, Christe P (2014) The effect of host social system on parasite population genetic structure: comparative population genetics of two ectoparasitic mites and their bat hosts. BMC Evolutionary Biology 14, 18 https://doi.org/10.1186/1471-2148-14-18
Dekeukeleire D, Janssen R, van Schaik J (2013). Frequent melanism in Geoffroy’s bat (Myotis emarginatus, Geoffroy 1806). Hystrix, the Italian Journal of Mammalogy, 24(2), 197-198. https://doi.org/10.4404/hystrix-24.2-8770
Kerth G, Fleischmann D, van Schaik J, Melber M (2013) From behaviour and genetics to nature conservation: 20 years research on Bechstein’s bats. In: Populationsökologie und Habitatansprüche der Bechsteinfledermaus Myotis bechsteinii (ed. Dietz M), pp. 35-50. Zarbock GmbH & Co. KG, Frankfurt.
Kerth G, van Schaik J (2012) Causes and consequences of living in closed societies: lessons from a long-term socio-genetic study on Bechstein's bats. Molecular Ecology 21, 633-646 https://doi.org/10.1111/j.1365-294X.2011.05233.x
van Schaik J, Bruyndonckx N, Kerth G, Christe P (2011) Isolation and characterisation of microsatellite loci for two species of Spinturnicid bat wing mites (Spinturnix myoti and Spinturnix bechsteini). Acarologia 51, 127-131 https://doi.org/10.1051/acarologia/20111997
Coyer JA, Hoarau G, van Schaik J, Luijckx P, Olsen JL (2011) Trans‐Pacific and trans‐Arctic pathways of the intertidal macroalga Fucus distichus L. reveal multiple glacial refugia and colonizations from the North Pacific to the North Atlantic. Journal of Biogeography 38, 756-771 https://doi.org/10.1111/j.1365-2699.2010.02437.x
Other publications
van der Plas ALD, van Schaik J (2010) Determinatie tabel tot de boom- en bodemwantsen van Nederland [translation: Key to the stink bugs of Netherlands]. Jeugdbondsuitgeverij. ISBN: 978-90-5107-044-6

Dr. Jaap van Schaik
Applied Zoology and Nature Conservation
Loitzer Str. 26
17489 Greifswald
Tel.: +49 (0)3834 420-4068
Fax: +49 (0)3834 420-4252
vanschaika(at)uni-greifswald(dot)de
I use a broad range of techniques to study the life history, social system, host-parasite interactions and population dynamics of European bat species.
Like nearly all taxa, European bat populations currently face a daunting diversity of anthropogenic threats (including habitat fragmentation, reductions in roost availability and prey density, expansion of wind energy, climate change). Given their slow life history, even small increases in mortality rate can dramatically impact populations. Therefore, the need for accurate estimates of population dynamics and a fundamental understanding of how these effects differ between species has never been more pressing. However, due to their small size, their ability to fly, and their often cryptic nature, observing and monitoring bat populations requires creative solutions.

In this context, European bats offer an ideal comparative model system to explore the effects that social system has on life history and the potential consequences of environmental change. Nearly all temperate zone bat species follow a three-phase annual cycle. Female bats form summer maternity colonies. Male bats are often solitary, or form separate bachelor groups during this time. In autumn, mating takes place, either in temporary harems where males attract females to roosts that they defend, or at underground hibernacula in a promiscuous, lek-like mating system, known as swarming. During winter, individuals hibernate, either solitarily or in clusters. While all species broadly follow this cycle, their social systems nevertheless differ considerably. For example, maternity colony size may range from several, to several thousand individuals depending on the species. Likewise, species differ markedly in the degree to which individuals disperse, or interact with conspecifics from other colonies.
Light barriers and camera traps
Light barriers coupled with camera traps installed at the entrance to a hibernation sites, offer a unique non-invasive methodology to quantify all movements in and out of a site at unprecedented accuracy, resolution and scale. Together with Gabi Krivek, I am developing analysis tools around this method, including standardized analysis pipelines and a deep-learning solution for automated species identification (BatNet). With these we can monitor population dynamics at underground sites, and characterize the environmental drivers of hibernation phenology in different bat species.
Selected publications:
Krivek G, Gillert A, Harder M, Fritze M, Frankowski K, Timm L, Meyer-Olbersleben L, von Lukas UF, Kerth G, van Schaik J (2023). BatNet: a deep learning-based tool for automated bat species identification from camera trap images. Remote Sensing in Ecology and Conservation http://doi.org/10.1002/rse2.339
Krivek G, Mahecha EPN, Meier F, Kerth G, & van Schaik J (2023). Counting in the dark: estimating population size and trends of bat assemblages at hibernacula using infrared light barriers. Animal Conservationhttp://doi.org/10.1111/acv.12856
Krivek G, Schulze B, Poloskei PZ, Frankowski K, Mathgen X, Douwes A, & van Schaik J (2022). Camera traps with white flash are a minimally invasive method for long‐term bat monitoring. Remote Sensing in Ecology and Conservation 8(3), 284-296 https://doi.org/10.1002/rse2.243
Movement ecology and population (genetic) structure
The behavior and movement ecology of bats, and particularly the timing of the transitions between the three phases of the annual cycle remain poorly characterized. To investigate these aspects, we use a wide variety of techniques, including time-series captures and counts at underground sites, radio telemetry, RFID-tags and loggers, and genetic sampling. Current questions include how juvenile bats find suitable hibernation sites, the degree of site fidelity of bats at hibernation sites (both together with Frauke Meier and Prof. Gerald Kerth), as well as quantifying the dispersal rate and population genetic structure of several bat species including Bechstein’s bat (with Prof. Gerald Kerth), lesser horseshoe bat (with Thomas Näf and Antonia Hammer), barbastelle bat (with Moritz Krämer), and Nathusius’s pipistrelle.
Selected publications:
Reusch C, Scheuerlein A, Grosche L, Meier F, Gampe J, Dammhahn M, van Schaik J & Kerth G (2023). The risk faced by the early bat: individual plasticity and mortality costs of the timing of spring departure after hibernation. Oikos e09654 http://doi.org/10.1111/oik.09654
Meier F, Grosche L, Reusch C, Runkel V, van Schaik J§, & Kerth G§ (2022). Long-term individualized monitoring of sympatric bat species reveals distinct species-and demographic differences in hibernation phenology. BMC Ecology and Evolution22(7), 1-12 https://doi.org/10.1186/s12862-022-01962-6
Stumpf M, Meier F, Grosche L, Halczok T, van Schaik J, Kerth G (2017) Do young bats follow their mothers to swarming sites? Results from a genetic maternity assignments of two bat species at a large hibernaculum in Germany. Acta Chiropterologica 19, 319-327 https://doi.org/10.3161/15081109ACC2017.19.2.008
Dekeukeleire D, Janssen R, Haarsma A-J, Bosch T, van Schaik J (2016) Swarming Behaviour, Catchment Area and Seasonal Movement Patterns of the Bechstein's Bats: Implications for Conservation. Acta Chiropterologica 18, 349-358 https://doi.org/10.3161/15081109acc2016.18.2.004
van Schaik J, Janssen R, Bosch T, Haarsma A-J, Dekker JJA, Kranstauber B (2015) Bats swarm where they hibernate: compositional similarity between autumn swarming and winter hibernation assemblages at five underground sites. PloS one 10.7 e0130850 https://doi.org/10.1371/journal.pone.0130850
Kerth G, van Schaik J (2012) Causes and consequences of living in closed societies: lessons from a long-term socio-genetic study on Bechstein's bats. Molecular Ecology 21, 633-646 https://doi.org/10.1111/j.1365-294X.2011.05233.x
Host-parasite evolutionary dynamics
In addition to the bats themselves, the differences in mating system and social organization seen in European bats offer an ideal comparative framework to explore how such differences in host sociality affect the transmission and genetic structure of their obligate ectoparasites and pathogens. I use comparative population genetics and time series sampling of ectoparasites from wild bat populations to investigate how variation in social system interact with parasite life-history characteristics to shape the transmission dynamics, and the broad- and local-scale population genetic structure of the parasites. This work focuses primarily on Spinturnicid wing-mites and Nycteribid bat flies, as well as a pathogen that is vectored by the bat flies: haemosporidians of the genus Polychromophilus (Together with Dr. Juliane Schaer, Humboldt University Berlin and Branka Bajíc, University of Belgrade, Serbia). In addition, I am using similar techniques to investigate the prevalence and genetic diversity of tick-borne pathogens in Sifakas together with Dr. Rebecca Lewis (University of Texas-Austin).
Selected publications:
Bajić B, Werb O, Budinski I, Blagojević J, Schaer J§, van Schaik J§ (2023) Non-invasive investigation of Polychromophilus parasite infections in bat populations in Serbia using bat flies. Parasites & Vectors
Pejić B, Budinski I, van Schaik J§, & Blagojević J§ (2022). Sharing roosts but not ectoparasites: high host-specificity in bat flies and wing mites of Miniopterus schreibersii and Rhinolophus ferrumequinum (Mammalia: Chiroptera). Current Zoology 68(5), 507-516 https://doi.org/10.1093/cz/zoab086
van Schaik J, Dekeukeleire D, Gazaryan S, Natradze I, Kerth G (2018) Comparative phylogeography of a vulnerable bat and its ectoparasite reveals dispersal of a non-mobile parasite among distinct evolutionarily significant units of the host. Conservation Genetics 19, 481-494 https://doi.org/10.1007/s10592-017-1024-9
van Schaik J, Kerth G (2017) Host social organization and mating system shape parasite transmission opportunities in three European bat species. Parasitology Research 116, 589-599 https://doi.org/10.1007/s00436-016-5323-8
van Schaik J, Dekeukeleire D, Kerth G (2015) Host and parasite life history interplay to yield divergent population genetic structures in two ectoparasites living on the same bat species. Molecular Ecology 24, 2324-2335 https://doi.org/10.1111/mec.13171
van Schaik J, Kerth G, Bruyndonckx N, Christe P (2014) The effect of host social system on parasite population genetic structure: comparative population genetics of two ectoparasitic mites and their bat hosts. BMC Evolutionary Biology 14, 18 https://doi.org/10.1186/1471-2148-14-18
- Gabriella Krivek: Automatic image-based species identification of European bats using deep learning algorithms (part of the DIG-IT! Landesexzellenzprogramm MV)
- Thomas Näf: Range expansion and dispersal in lesser horseshoe bats (GRK 2010 Biological RESPONSEs to Novel and Changing Environments), 2nd Cohort
- Antonia Hammer: Range expansion and dispersal in lesser horseshoe bats (GRK 2010 Biological RESPONSEs to Novel and Changing Environments), 3rd Cohort
- Frauke Meier: Localization and use of hibernacula in temperate zone bats
- Moritz Krämer: Population genetic structure and range expansion of the Barbastelle bat (Barbastella barbastellus) in Germany (Part of the Verbundprojekt: “Schutz und Förderung der Mopsfledermaus in Deutschland“, https://www.mopsfledermaus.de )
Samuel Schuler (MSc): title TBA
Benjamin Dötterl (MSc): title TBA
Former students: Luisa Timm (MSc), Karina Frankowski (MSc), Anja Fritzsche (MSc), Laura Maaß (MSc), Lucie Hoffschläger (BSc), Daan Dekeukeleire (MSc)
MSc BEE Module E1.9 & E1.10 / MSc LENC Module E24 & E25; Every WiSe
Conservation and Landscape Genetics (Lecture)
Current topics in Conservation Genetics (Seminar)
Applied Conservation Genetics (Practical)
Google Scholar: https://scholar.google.de/citations?user=acnwIxQAAAAJ
ORCID: 0000-0003-4825-7676
§ indicates shared first/senior authorship
Krivek, G., Meier, F., Grosche, L., Kerth, G.§ , & van Schaik, J.§ (2025). One Species Hibernates Shorter, the Other Longer: Rapid but Opposing Responses to Warming Climate in Two Sympatric Bat Species. Global Change Biology 31, no. 10: e70531. https://doi.org/10.1111/gcb.70531.
Meier, F., Grosche, L., Krivek, G., Runkel, V., Scheuerlein, A., Kerth, G., & van Schaik, J. (2025). Automated long‐term monitoring of RFID‐tagged individuals reveals high hibernaculum site fidelity in Daubenton's bats and Natterer's bats. Animal Conservation, 28(3), 401-409. https://doi.org/10.1111/acv.12992
van Schaik, J., Schuler, S., Stienstra, K., Janssen, R., Dekeukeleire, D., Boshamer, J.P., Noort, B.C., Steenbergen, J. and Lagerveld, S. (2025). Diverse but declining? Population genetic structure and genetic diversity of Nathusius’ pipistrelle along the Dutch coastline during the autumn migration period. Mammalian Biology, 1-12. https://doi.org/10.1007/s42991-025-00486-y
Timm, L., Rosskopf, S. P., Werb, O., van Schaik, J., & Schaer, J. (2025). Europe-wide distribution and bat-host specific lineages in the malarial parasite Polychromophilus murinus revealed through genetic screening of bat flies. Infection, Genetics and Evolution, 127, 105707. https://doi.org/10.1016/j.meegid.2024.105707
Mundinger C, van Schaik J, Scheuerlein A & Kerth G (2023). Heat over heritability: Increasing body size in response to global warming is not stabilized by genetic effects in Bechstein's bats. Global Change Biology, 00, 1– 10. https://doi.org/10.1111/gcb.16824
Bajić B, Werb O, Budinski I, Blagojević J, Schaer J§, van Schaik J§ (2023) Non-invasive investigation of Polychromophilus parasite infections in bat populations in Serbia using bat flies. Parasites & Vectors
Krivek G, Gillert A, Harder M, Fritze M, Frankowski K, Timm L, Meyer-Olbersleben L, von Lukas UF, Kerth G, van Schaik J (2023). BatNet: a deep learning-based tool for automated bat species identification from camera trap images. Remote Sensing in Ecology and Conservation http://doi.org/10.1002/rse2.339
Naef T, Besnard, A-L, Lehnen L, Petit EJ, van Schaik J§, Puechmaille SJ§ (2023). How to quantify factors degrading DNA in the environment and predict degradation for effective sampling design. Environmental DNA http://doi.org/10.1002/edn3.414
Krivek G, Mahecha EPN, Meier F, Kerth G, & van Schaik J (2023). Counting in the dark: estimating population size and trends of bat assemblages at hibernacula using infrared light barriers. Animal Conservation http://doi.org/10.1111/acv.12856
Reusch C, Scheuerlein A, Grosche L, Meier F, Gampe J, Dammhahn M, van Schaik J & Kerth G (2023). The risk faced by the early bat: individual plasticity and mortality costs of the timing of spring departure after hibernation. Oikos e09654 http://doi.org/10.1111/oik.09654
Kerth G, van Schaik J (2022). Bechstein’s Bat Myotis bechsteinii (Kuhl, 1817). In: Hackländer, K., Zachos, F.E. (eds) Handbook of the Mammals of Europe. Handbook of the Mammals of Europe. Springer, Cham. doi.org/10.1007/978-3-319-65038-8_58-1
Kappeler PM, Benhaiem S, Fichtel C, Fromhage L, Höner OP, Jennions MD, Kaiser S, Krüger O, Schneider JM, Tuni C, van Schaik J & Goymann W (2022). Sex roles and sex ratios in animals. Biological Reviews 98: 462-480 https://doi.org/10.1111/brv.12915
Meier F, Grosche L, Reusch C, Runkel V, van Schaik J§, & Kerth G§ (2022). Long-term individualized monitoring of sympatric bat species reveals distinct species-and demographic differences in hibernation phenology. BMC Ecology and Evolution22(7), 1-12 https://doi.org/10.1186/s12862-022-01962-6
Pejić B, Budinski I, van Schaik J§, & Blagojević J§ (2022). Sharing roosts but not ectoparasites: high host-specificity in bat flies and wing mites of Miniopterus schreibersii and Rhinolophus ferrumequinum (Mammalia: Chiroptera). Current Zoology 68(5), 507-516 https://doi.org/10.1093/cz/zoab086
Krivek G, Schulze B, Poloskei PZ, Frankowski K, Mathgen X, Douwes A, & van Schaik J (2022). Camera traps with white flash are a minimally invasive method for long‐term bat monitoring. Remote Sensing in Ecology and Conservation 8(3), 284-296 https://doi.org/10.1002/rse2.243
Stapelfeldt B, Schöner M, Kerth G, & van Schaik J (2020). Slight increase in bat activity after human hibernation count monitoring of a bunker complex in northern Germany. Acta Chiropterologica 22(2), 383-390 https://doi.org/10.3161/15081109acc2020.22.2.012
Gill LF, van Schaik J, von Bayern AM, & Gahr ML (2020). Genetic monogamy despite frequent extrapair copulations in “strictly monogamous” wild jackdaws. Behavioral Ecology 31, 247-260 https://doi.org/10.1093/beheco/arz185
van Schaik J, Dekeukeleire D, Gazaryan S, Natradze I, Kerth G (2018) Comparative phylogeography of a vulnerable bat and its ectoparasite reveals dispersal of a non-mobile parasite among distinct evolutionarily significant units of the host. Conservation Genetics 19, 481-494 https://doi.org/10.1007/s10592-017-1024-9
Stumpf M, Meier F, Grosche L, Halczok T, van Schaik J, Kerth G (2017) Do young bats follow their mothers to swarming sites? Results from a genetic maternity assignments of two bat species at a large hibernaculum in Germany. Acta Chiropterologica 19, 319-327 https://doi.org/10.3161/15081109ACC2017.19.2.008
van Schaik J, Kerth G (2017) Host social organization and mating system shape parasite transmission opportunities in three European bat species. Parasitology Research 116, 589-599 https://doi.org/10.1007/s00436-016-5323-8
Dekeukeleire D, Janssen R, Haarsma A-J, Bosch T, van Schaik J (2016) Swarming Behaviour, Catchment Area and Seasonal Movement Patterns of the Bechstein's Bats: Implications for Conservation. Acta Chiropterologica 18, 349-358 https://doi.org/10.3161/15081109acc2016.18.2.004
van Schaik J, Janssen R, Bosch T, Haarsma A-J, Dekker JJA, Kranstauber B (2015) Bats swarm where they hibernate: compositional similarity between autumn swarming and winter hibernation assemblages at five underground sites. PloS one 10.7 e0130850 https://doi.org/10.1371/journal.pone.0130850
van Schaik J, Dekeukeleire D, Kerth G (2015) Host and parasite life history interplay to yield divergent population genetic structures in two ectoparasites living on the same bat species. Molecular Ecology 24, 2324-2335 https://doi.org/10.1111/mec.13171
van Schaik J, Kerth G, Bruyndonckx N, Christe P (2014) The effect of host social system on parasite population genetic structure: comparative population genetics of two ectoparasitic mites and their bat hosts. BMC Evolutionary Biology 14, 18 https://doi.org/10.1186/1471-2148-14-18
Dekeukeleire D, Janssen R, van Schaik J (2013). Frequent melanism in Geoffroy’s bat (Myotis emarginatus, Geoffroy 1806). Hystrix, the Italian Journal of Mammalogy, 24(2), 197-198. https://doi.org/10.4404/hystrix-24.2-8770
Kerth G, Fleischmann D, van Schaik J, Melber M (2013) From behaviour and genetics to nature conservation: 20 years research on Bechstein’s bats. In: Populationsökologie und Habitatansprüche der Bechsteinfledermaus Myotis bechsteinii (ed. Dietz M), pp. 35-50. Zarbock GmbH & Co. KG, Frankfurt.
Kerth G, van Schaik J (2012) Causes and consequences of living in closed societies: lessons from a long-term socio-genetic study on Bechstein's bats. Molecular Ecology 21, 633-646 https://doi.org/10.1111/j.1365-294X.2011.05233.x
van Schaik J, Bruyndonckx N, Kerth G, Christe P (2011) Isolation and characterisation of microsatellite loci for two species of Spinturnicid bat wing mites (Spinturnix myoti and Spinturnix bechsteini). Acarologia 51, 127-131 https://doi.org/10.1051/acarologia/20111997
Coyer JA, Hoarau G, van Schaik J, Luijckx P, Olsen JL (2011) Trans‐Pacific and trans‐Arctic pathways of the intertidal macroalga Fucus distichus L. reveal multiple glacial refugia and colonizations from the North Pacific to the North Atlantic. Journal of Biogeography 38, 756-771 https://doi.org/10.1111/j.1365-2699.2010.02437.x
Other publications
van der Plas ALD, van Schaik J (2010) Determinatie tabel tot de boom- en bodemwantsen van Nederland [translation: Key to the stink bugs of Netherlands]. Jeugdbondsuitgeverij. ISBN: 978-90-5107-044-6